The Endpoint
A skin sensitiser is a substance that will induce an allergic response following skin contact. Substances are classed as skin sensitisers:
- if there is evidence in humans that the substance can induce sensitisation by skin contact in a substantial number of persons, or
- where there are positive results from an appropriate animal test.
A range of in vivo methods exist that has been proven to be very accurate in terms of the predictive identification of chemicals that possess skin sensitizing properties. For many years, guinea pigs were the species of choice for sensitisation tests. Two types of tests were developed; adjuvant tests in which sensitisation is potentiated by the injection of Freund’s Complete Adjuvant (GPMT), and non adjuvant tests (The Buehler test).
The Local Lymph Node Assay (LLNA) is based upon the characteristics of induced proliferative responses in draining lymph nodes following topical exposure of chemicals to mice. The endpoint is the stimulation index (SI), which gives a ratio of thymidine incorporation in lymph nodes from dosed animals compared to the incorporation in lymph nodes from vehicle treated control animals. The test is positive when the stimulation index (SI) is greater than 3 for any of the dose concentrations. The EC3 value, interpolated from the dose response curve, is the effective concentration of the test substance required to produce a three-fold increase in the stimulation index compared to vehicle-treated controls.
Furthermore progress in understanding the mechanisms of skin sensitization, including effects on the production of cytokines by the different cell types within the skin, provides the opportunity to develop in vitro tests as an alternative to in vivo sensitization testing. With the forthcoming elimination of in vivo tests for cosmetic testing in the European Union, several opportunities that have been exploited for in vitro test development focus on key elements of the sensitization process. One unifying characteristic of chemical allergens is the requirement that they react with proteins for the effective induction of skin sensitization. The majority of chemical allergens are electrophilic and react with nucleophilic amino acids.
Skin Sensitization & REACH
Skin sensitization is an endpoint that needs to be assessed within REACH. The test of first choice under REACH for skin sensitisation is the Local Lymph Node Assay (LLNA).
According to OECD data, testing for skin sensitisation accounts for over 5% of the total use of animal tests. OECD guideline no. 406 recommends the use of 30 animals (guinea pigs) while OECD guideline no. 429 recommend the use of 25 animals (mouse) for the LLNA assay.
In REACH, sensitising potential needs to be assessed for chemicals above the 1 ton threshold according to Annex VII (EC, 2006a).
QSAR & Skin Sensitization for REACH
One potential alternative approach to skin sensitisation hazard identification is the use of (quantitative) structure activity relationships (QSARs) coupled with appropriate documentation and performance characteristics. This represents a major challenge. Current thinking is that QSARs might best be employed as part of a battery of approaches that collectively provide information on skin sensitisation hazard.
The Approach
To address the skin sensitization issue two complementary approaches were investigated:
- A global approach aimed at developing a classifier to discriminate sensitizers vs non sensitizers
- A local approach that investigated a mechanistic based category formation coupled with a read-across approach within each category
The Model
Global QSAR model
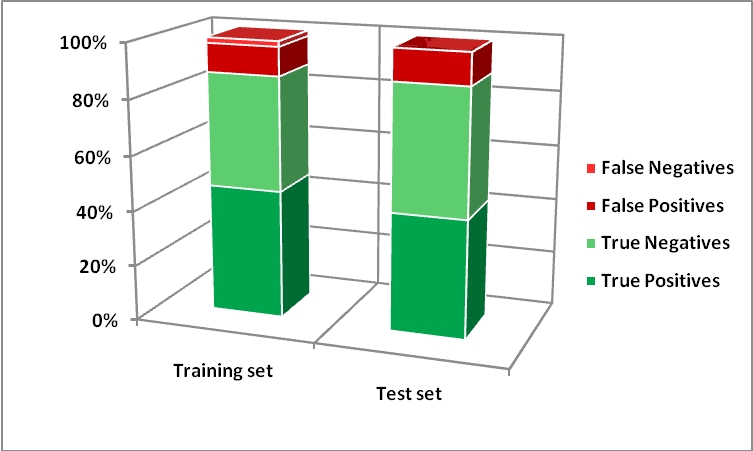
This CAESAR model for skin sensitization is based on a data set that includes 209 compounds extracted from the paper by Gerberick et al. For developing classification models, this data set was subdivided in two classes, sensitiser (S) and non-sensitisers (N), which gave a good distribution of the numbers of compounds in each class. The class S merges the first four ranges established by ECETOC: Extreme (EC3<0.1%), Strong (0.1%<EC3<1%), and Moderate (1%<EC3<10%) and Weak (EC3>10%) ranges; the class N regroups all compounds belonging to the non sensitisers. The data set was subdivided into a training set (about 80% of the compounds) used to develop the model and a test (including the remaining 20% of the data) used to assess the performance of the model in prediction.
| EC3 (%) | LLNA Class |
|---|---|
| NC | Non Sensitiser |
| ≥10 | Weak Sensitiser |
| 1-10 | Moderate Sensitiser |
| 0.1-1 | Strong Sensitiser |
| <0.1 | Extreme Sensitiser |
The CAESAR model for skin sensitization was developed using Adaptive Fuzzy Partition (AFP) – AFP was used to develop classification models implementing a fuzzy partition algorithm. It models relations between molecular descriptors and chemical activities by dynamically dividing the descriptor space into a set of fuzzy partitioned subspaces. The aim of this algorithm is to select the descriptor and the cut position that allow to get the maximal difference between the two fuzzy rule scores generated by the new subspaces. The score is determined by the weighted average of the chemical activity values in an active subspace A and in its neighbouring subspaces.
This model was derived from 8 descriptors from Dragon software (nN; GNar; MDDD; X2v; EEig10r; GGI8; nCconj; O-058). For the feature selection, a hybrid selection algorithm (HSA), which combines the genetic algorithm (GA) concepts and a stepwise approach, was used to select the best descriptors for classifying developmental toxicity dataset.
Results of the binary classification model are shown below:
Local QSAR model
A complementary approach was developed to enable potential mechanisms of toxic action to be assigned to chemicals thought to be capable of skin sensitisation. This approach was based on the work of Aptula and Roberts [2] who had previously suggested that for a chemical to be a skin sensitiser it must be capable (either directly or after some abiotic or metabolic transformation) of one of five electrophilic-nucleophilic reactions. The approach undertaken was to devise SMARTS patterns capable of identifying the electrophilic mechanisms previously assigned to the 210 Gerberick LLNA dataset [3]. The 44 chemical TIMES-SS LLNA data, in which each chemical has also had a mechanism of action assigned to it by the same authors [4] was then used to validate the applicability of the SMARTS patterns.
The table below show the confusion matrix for the TIMES-SS validation set of chemicals. Expert assignment from reference [4].
| non | 23 | ||||
| Ac | pro-MA | SB | SN2 | non | |
|---|---|---|---|---|---|
| Ac | 2 | ||||
| pro-MA | 5 | 4 | |||
| SB | 4 | ||||
| SN2 | 6 |
The results of this approach are the subject of a forthcoming publication [5]. The table below represents the confusion matrix showing the classification statistics for the training data. The columns indicate the expert assignment from reference [3]; the rows report the class from the SMARTS pattern.
| non | 22 | |||||||||
| Ac | MA | pro-MA | SB | pro-SB | SN2 | pro-SN2 | SNAr | SN1 | non | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ac | 24 | 1 | 1 | |||||||
| MA | 29 | 2 | ||||||||
| pro-MA | 25 | |||||||||
| SB | 36 | 3 | ||||||||
| pro-SB | 4 | |||||||||
| SN2 | 2 | 2 | 45 | |||||||
| pro-SN2 | 2 | |||||||||
| SNAr | 3 | |||||||||
| SN1 | 1 |
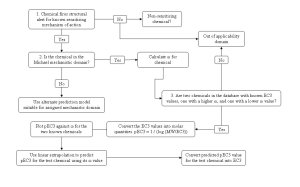
The ability to assign a chemical to one of the suggested electrophilic mechanisms allowed for the development of a novel quantitative read-across approach for the prediction of skin sensitising potential of chemicals assigned to the Michael mechanistic domain (see figure below).
This approach utilised the electrophilic index (ω) which has been suggested to account for a chemical’s inherent electrophilicity [6]. This descriptor was calculated from the frontier molecular orbitals, these are the orbitals considered to be directly involved in the formation of the covalent bond between the nucleophilic sulphur containing protein side chains and the electrophilic chemical thought to be the initial event required for skin sensitisation. Examples of the predictions obtained by the method can be seen in the table below and full details of how to carry out the calculations and predictions for the remaining chemicals assigned to the Michael domain can be found in reference [7].
| Name | Structure | Experimental EC3 | Predicted EC3 | ω |
|---|---|---|---|---|
| trans-2-hexenal |  | 5.5 | NP | 1.608 |
| 1-(4-methoxyphenyl)-1-penten-3-one | 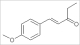 | 9.3 | 9.87 | 1.734 |
| safranal (1,1,3-trimethyl-2-formylcyclohexa-2,4-diene) | 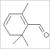 | 7.5 | 5.29 | 1.796 |
| diethyl maleate |  | 5.8 | 8.74 | 1.804 |
| α-amyl cinnamic aldehyde | 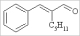 | 11 | NP | 1.839 |
NP means a prediction has not been made is not a chemical more electrophilic (larger ω) or less electrophilic (smaller ω) in this small.
References
- Global QSAR models of skin sensitisers for regulatory purposes.
Chaudhry Q., Piclin N., Cotterill J., Pintore M., Price N.R., Chrétien J.R., Roncaglioni A.
Chemistry Central Journal 2010, 4(Suppl 1):S5 (29 July 2010) - Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods
Gerberick, G.F., C.A. Ryan, P.S. Kern, et al.
Dermatitis, 2005;16:157-202. - Skin sensitization: Reaction mechanistic applicability domains for structure-activity relationships.
Aptula, A.O., G. Patlewicz, and D.W. Roberts.
Chemical Research in Toxicology, 2005;18:1420-1426. - Mechanistic Applicability Domain Classification of a Local Lymph Node Assay Dataset for Skin Sensitization.
Roberts, D.W., G. Patlewicz, P.S. Kern, et al.
Chemical Research in Toxicology., 2007;20:1019-1030. - TIMES-SS - A mechanistic evaluation of an external validation study using reaction chemistry principles.
Roberts, D.W., G. Patlewicz, S.D. Dimitrov, et al.
Chemical Research in Toxicology, 2007;20:1321-1330. - Identification of mechanisms of toxic action for skin sensitisation using a SMARTS pattern based approach.
Enoch, S. J., J. C. Madden, and Cronin, M. T. D.
SAR and QSAR in Environmental Research, 2008;19(5-6):555-78. - Electronegativity - Density Functional Viewpoint.
Parr, R.G., R.A. Donnelly, M. Levy, et al.
Journal of Chemical Physics, 1978;68:3801-3807. - Quantitative and Mechanistic Read Across for Predicting the Skin Sensitization Potential of Alkenes Acting via Michael Addition.
Enoch, S.J., M.T.D. Cronin, T.W. Schultz, et al.
Chemical Research in Toxicology, 2008;21:513-520.