REACH is a very complex legislation facing a huge task: to protect human health and environment, regulating chemical substances. To do this, a series of data have to be gathered, and we should not lose any possible information. REACH promotes the use of many methods, both to save animals, and to increase the information robustness.
The so-called alternative methods, including the in silico and QSAR models, have an important role within REACH to better study the effects of chemical substances.
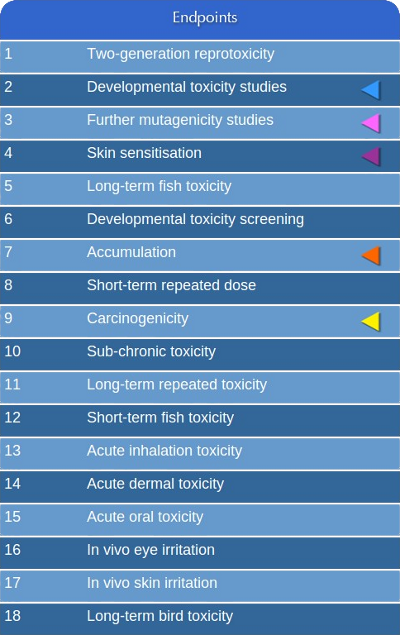
In CAESAR we addressed with (Q)SAR models five of the endpoints defined
within the list of priority regulatory endpoints(*).
The endpoints have been chosen
using two criteria: 1) addressing those endpoints that needs the highest number of animal testing
(in vivo testing); 2) availability of high quality experimental data to use as
training set for building the predictive models.
(*) As defined in: Van der Jagt et al. (2004) Alternative approaches can reduce the use of test animals under REACH. Addendum to the report “Assessment of additional testing needs under REACH. Effects of (Q)SARs, risk based testing and voluntary industry initiatives”.
For more info on REACH, please visit:
- Legislation (full text PDF)
http://eur-lex.europa.eu/LexUriServ/site/en/oj/2006/l_396/l_39620061230en00010849.pdf - DG Enterprise
http://europa.eu.int/comm/enterprise/reach - DG Environment
http://europa.eu.int/comm/environment/chemicals/reach.htm