The Endpoint
Developmental toxicity includes any effect interfering with normal development, both before and after birth. It includes effects induced or manifested either pre- or postnatally. This includes embryotoxic/foetotoxic effects such as reduced body weight, growth and developmental retardation, organ toxicity, death, abortion, structural defects (teratogenic effects), functional effects, peri- and postnatal defects, and impaired postnatal mental or physical development up to and including normal pubertal development.
Information Requirement
The standard data requirements for developmental toxicity under the REACH Regulations are as follows:
- A reproduction/developmental toxicity screening test (OECD TGs 421 or 422), usually required for substances produced or imported in quantities between 10 and 100 tons/year (according to Annex VIII of the REACH legislation).
- A prenatal developmental toxicity study (EU B.31, OECD TG 414) in one species, usually required for substances produced or imported in quantities of ≥100 tons/year (according to Annex IX and X of the REACH legislation). A study in a second species may be considered necessary at these levels of production/importation.
However, these tonnage-related standard data requirements may be adapted, either reduced (a data waiver), deferred or extended. Factors that can influence the testing requirements include structural relationships with other chemicals, the results of other toxicity studies, presence of mutagenic and carcinogenic properties, available data from humans exposed to the substance, concerns for endocrine disruption and the patterns of use and human exposure.
Developmental Effects
The golden standard test under REACH for developmental toxicity is OECD 414, which recommends the use of 80 adult animals (rats or rabbits) for this study. Studies according to OECD 421 or 422 are used for screening only. If positive, they may not be considered sufficient for classification & labeling and quantitative risk assessment. If negative for a substance produced or imported in quantities between 10 and 100 tons/year, no further testing is needed. A negative outcome of these screening studies for substances produced/imported in quantities over 100 tons/year, implies a OECD 414 study needs to be performed.
Hazard Assessment
Developmental toxicity may be identified from epidemiological studies, from animal experiments and/or other appropriate means that may include (Quantitative) Structure-Activity Relationships ((Q)SAR) analyses and/or extrapolation from structurally similar substances (read-across). Developmental effects should be considered in relation to adverse effects occurring in the parents. Since adverse effects in pregnancy or postnatally may result as a secondary consequence of maternal toxicity, reduced food or water intake, maternal stress, lack of maternal care, specific dietary deficiencies, poor animal husbandry, intercurrent infections etc., it is important that the effects observed should be interpreted in conjunction with possible concomitant maternal toxicity. The nature, severity and dose-response of all effects observed in progeny and parental animals should be considered and compared together to achieve a balanced integrated assessment of available data on all endpoints relevant for developmental toxicity.
The Model
The dataset extracted from Arena et al. (2004) includes 292 compounds divided into a training and a test set.
Chemical compounds were categorized into toxicant or non toxicant according to FDA risk factors.
| FDA classes | Definition | CAESAR Binary class |
|---|---|---|
| Category A | Negative human studies | Non developmental toxicant |
| Category B |
Negative animal studies &
No human studies executed OR Positive animal studies & Negative human studies |
|
| Category C | Postive animal studies &
No human studies executed OR No studies at all |
Developmental toxicant |
| Category D | Postive human studies | |
| Category X | Animal OR human studies show abnormalities AND/OR Evidence of foetal risk based on human experience |
The chemical descriptors have been calculated in collaboration with Dr Todd Martin, US EPA with a public US EPA program .
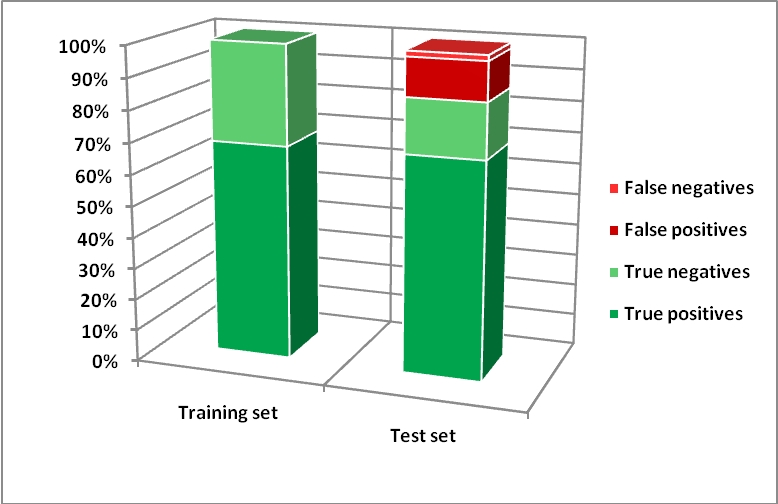
Several models have been developed: in one case (model A) the software used for the model is WEKA (Waikato Environment for Knowledge Analysis), an open source workbench. In this case we used 13 chemical descriptors.
The algorithm used for modeling is Random Forest that constructs a "forest" of random "trees" (this model has been implemented in the CAESAR software).
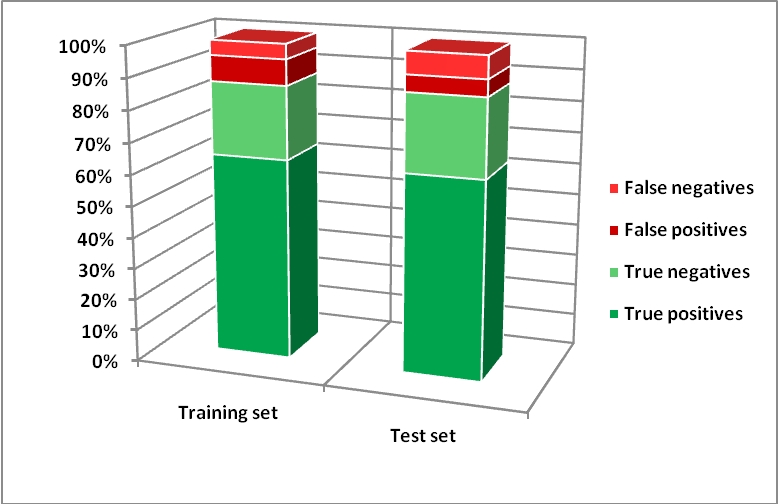
A second model (model B) was developed using Adaptive Fuzzy Partition (AFP) – AFP was used to develop classification models implementing a fuzzy partition algorithm. It models relations between molecular descriptors and chemical activities by dynamically dividing the descriptor space into a set of fuzzy partitioned subspaces. The aim of this algorithm is to select the descriptor and the cut position that allow to get the maximal difference between the two fuzzy rule scores generated by the new subspaces. The score is determined by the weighted average of the chemical activity values in an active subspace A and in its neighbouring subspaces.
In this case we used 6 chemical descriptors. For the feature selection, a hybrid selection algorithm (HSA), which combines the genetic algorithm (GA) concepts and a stepwise regression, was used to select the best descriptors for classifying developmental toxicity dataset.
Below there are the results for the two models on developmental toxicity dataset.
Notice for the use of in silico CAESAR models addressing human toxicology related to
human toxicology (i.e.: carcinogenicity and developmental toxicity models).
Currently, the role of in silico models in these endpoints can be limited to consider them as an
ingredient in deriving a weight of evidence rather than to substitute per se existing methods.
Their utility is consequently in support to the overall assessment.
The user is also advised that, since some of the models are based on datasets focused on a limited
chemical space, particular attention should be placed for these two endpoints in the evaluation of
similar compounds already present in the studied datasets and the model's ability to correctly
predict them.
QSAR Model Reporting Format
Download the QSAR Model Reporting Format for the CAESAR models.
This documentation provide all the information (including the dataset used to build and test the model) to judge the scientific validity of the models.
CLICK HERE TO DOWNLOAD
References
- CAESAR models for developmental toxicity.
Cassano A., Manganaro A., Martin T., Young D., Piclin N., Pintore M., Bigoni D., Benfenati E.
Chemistry Central Journal 2010, 4(Suppl 1):S4 (29 July 2010)